Stocking up for future use (I/II): how energy storage technologies work


· 7 min read
This is part one of a two-part series on energy storage technologies. You can find part two here.
In 2021, renewable sources — wind and solar — provided a record 10 percent of global electricity generation. By 2026, their combined capacity could increase by more than 60 percent. But giving up fossil fuels is still not that easy. If oil and gas stably provide us with energy, then, for example, the production of hydro, solar, and wind power plants largely depends on weather conditions. Energy accumulation and storage systems can eliminate this drawback.
Imagine living in a house that is powered entirely by solar panels. They convert the sun’s energy into electricity, saving nothing for the future. In good weather, such a system works great, but evening comes, and your home suddenly finds itself without power. The food in the refrigerator spoils, you have to use candles to light the room, and if you don’t have a power bank, then there’s no way to charge your smartphone or laptop.
Of course, in reality, there are always backup energy sources in the energy system — for example, a solar power plant without batteries can only be used during daylight hours, and in the absence of the sun, electricity can be used from the city network. However, this does not solve the global problem: alternative energy is still highly dependent on the weather. Meanwhile, the peak of energy consumption, as a rule, occurs in the morning and evening — just in the absence of sunlight. Millions of people get up in the morning for work and turn on electric kettles, and in the evening they watch TV or spend time playing video games. The situation is aggravated in autumn and winter when the sun can be hidden behind clouds for almost the whole day.
Peak loads can be compensated using non-renewable energy sources — coal or gas. But the point of alternative energy is to learn to do without them. It is worth considering the possibility of a failure in the power supply system. What to do if the weather is cloudy all week, the solar panels produce little energy, and the power plant has a failure just at that time?
In such a case, energy accumulation and storage systems can provide the house with light. They not only allow you to stock up on it for future use but also reduce the load on power plants. Storage helps compensate for the shortcomings of renewable sources, which do not produce energy consistently at the same rate or at exactly the times when people need it, and rely more on wind power and sunlight.
Storage systems eliminate the need for simultaneous energy production and consumption, but the principles of their operation can be very different.
Energy can be stored in chemical bonds that connect atoms in a molecule of a substance. Coal and oil are also, in a certain sense, a system for storing energy accumulated over millions of years of formation of deposits. However, fossil energy sources have a significant disadvantage: they are exhaustible.
The sun and wind do not have this disadvantage, and the energy obtained with their help can also be converted into chemical bonds. For example, send it to the electrolysis of water to produce hydrogen (this technology is called power-to-gas), which can be used as fuel. The gas is stored in special tanks and released as needed. When hydrogen fuel is burned, water is produced, thus completing the cycle.
Another way to convert wind or solar energy into chemical bonds is to use a rechargeable battery. Lithium-ion batteries have several advantages: they are quickly produced, store energy efficiently, and deliver it to devices almost instantly. Sony released its first batteries in 1991. Since then, their capacity has almost doubled, but progress has now slowed.
Any battery consists of two electrodes containing an oxidizing agent and a reducing agent, as well as a conducting medium — an electrolyte. In a discharged battery, the electrodes do not interact with each other, but under the influence of electric current they become unstable, acquiring excess oxidation and reduction potential. The electrodes begin to interact with each other, exchanging electrons, which can be passed through an external circuit and used, for example, to charge a smartphone. In a battery, chemical reactions are reversible. This means that they can be used over and over again.
Experts predict that the market for lithium-ion batteries will only grow: not only are they used in most compact gadgets such as smartphones and laptops, but they are also capable of powering electric vehicles and storing energy for utilities. Therefore, large corporations, in particular automakers, still trust this technology and invest in it. However, for such purposes, significantly more capacious batteries are required.
Such devices are produced, for example, by Tesla: the giant Megapack lithium-ion battery is designed for energy storage in power plants and utilities. The accumulated energy is consumed during peak load hours. And Mercedes-Benz Energy is developing similar systems based on used batteries and spare parts from electric vehicles.
Megapack batteries. Tesla

Still, lithium-ion batteries are not perfect. They are expensive to produce and quickly degrade and are also sensitive to damage and high temperatures. This not only leads to wear and tear on the batteries themselves but also causes fires that are difficult to extinguish: their combustion is accompanied by a violent chemical reaction. The U.S. Consumer Product Safety Commission said there were more than 25,000 reported problems involving lithium-ion batteries catching fire or overheating over five years.
For example, in 2016, 2 months after the start of sales, Samsung recalled more than 2.5 million Galaxy Note 7 smartphones due to numerous cases of fires and explosions — the damage amounted to approximately $5 billion. This was due to improper battery design. In 2021, a 13-ton Tesla Megapack battery caught fire at an energy storage facility under construction in Australia. A leak in the liquid cooling system caused a thermal runaway inside the battery modules, which led to a fire.
During the charging process of the battery, the electrolyte heats up, which leads to an increase in the charging current. Under certain conditions, it can rise to a value that approaches the short-circuit current, a phenomenon called thermal runaway. Its likelihood increases if the heat exchange between the battery and the environment is disrupted. In an effort to prevent batteries from catching fire, companies are adding systems to devices that monitor the health of the battery.
Scientists and engineers are working to overcome the shortcomings of lithium-ion batteries. For example, they teach them to turn off when overheated, build in a flame arrester, and try to use not carbon as an electrode, but a silicon-carbon nanocomposite. Lithium batteries with solid electrolytes and fast-charging batteries made from nanomaterials and organics are also being developed.
In addition to lithium-ion batteries, there are also lead-acid, sodium-sulfur, nickel-metal hydride, nickel-cadmium, and nickel-iron batteries, sodium-ion batteries, and aluminum batteries that fully charge in 45 minutes and do not burn. All of them differ from each other in service life, resistance to high and low temperatures, and rate of charge loss, as well as power and energy intensity. There is no universal drive — each of them is better suited to solve a specific problem.
Redox flow batteries are created for high-capacity stationary batteries. These are huge containers of electrolytes: vanadium, salt water, or a solution of chlorine or zinc, which is passed through a membrane and creates an electrical charge. Redox batteries are cheaper than rechargeable batteries, and their contents are not enclosed in a single housing, like batteries. This allows you to change the size of the electrolyte tanks, as well as the size of the modules that convert the energy of chemicals into electricity, and flexibly control power and capacity. Powerful flow batteries are now used in combination with solar and wind power plants.
Scheme of a redox flow battery
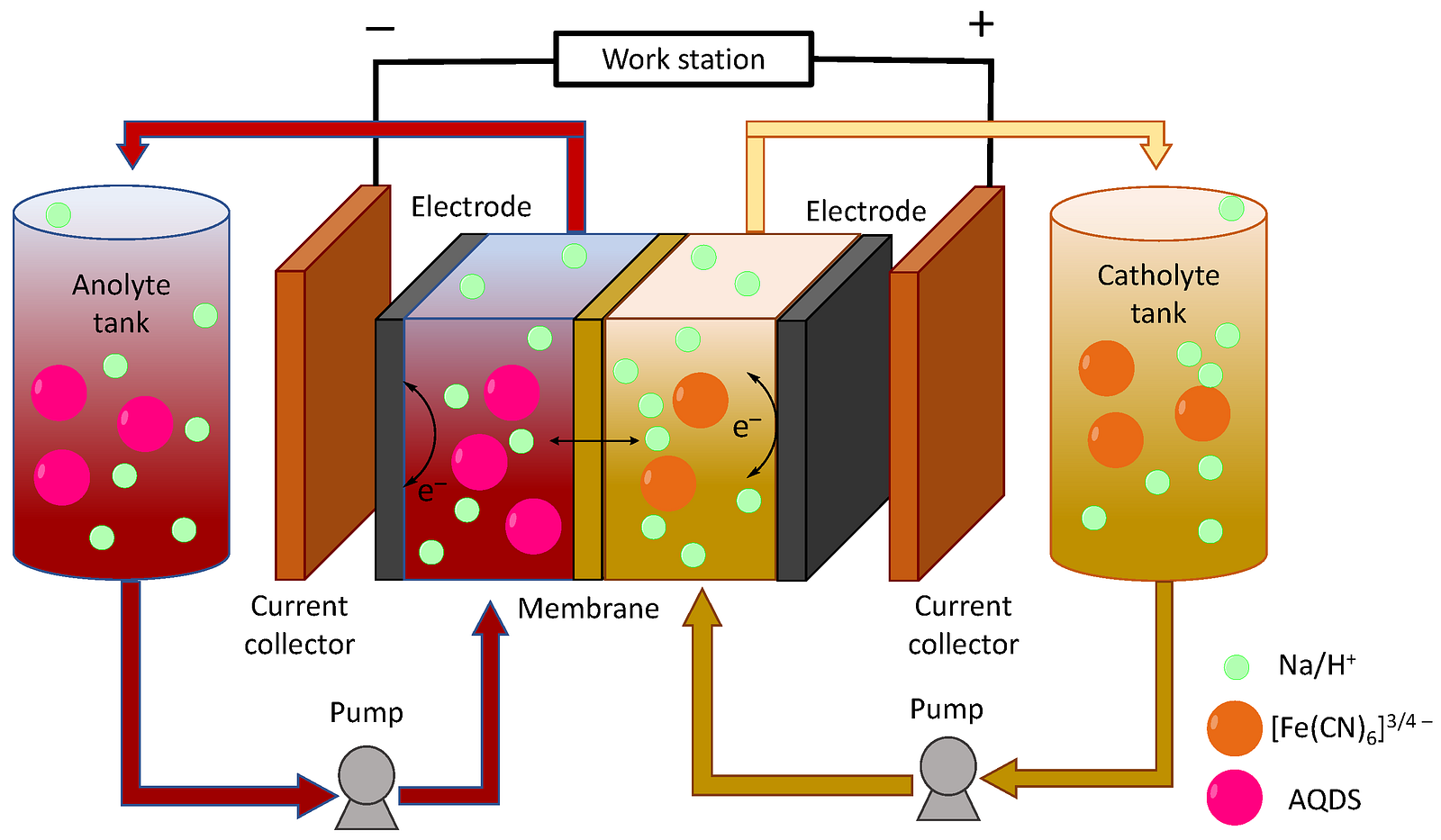
Another important development is supercapacitors. This is a device consisting of two electrodes immersed in an electrolyte and a separator that prevents charge movement between the electrodes. The special thing about supercapacitors is that they can charge in just a few seconds or minutes. Therefore, they are used where high power is required for a short period: to start the engine in cars, to reduce the load on the battery, in public transport (a trolleybus can bypass small areas on a supercapacitor where there is no contact network) and in consumer electronics — photo flashes and car radios.
Future Thought Leaders is a democratic space presenting the thoughts and opinions of rising Sustainability & Energy writers, their opinions do not necessarily represent those of illuminem.
illuminem briefings

Battery Tech · Green Tech
Yury Erofeev

Battery Tech · Mobility Tech
illuminem briefings

Battery Tech · Public Governance
The Wall Street Journal

Li-ion Battery · Battery Tech
Rolling Out

Battery Tech · Green Tech
Interesting Engineering

Power Grid · Battery